We often say to people that ultimately climate is a molecules game. The end game of climate action is to reduce the flow, and eventually the stock, of global warming gases in the atmosphere. The biggest opportunity to reduce emissions is through energy efficiency, and switching away from molecules and towards electrons for energy (whilst decarbonising electricity production). However, there are certain areas where electrons just won’t do, and we need to tackle these also. We use molecules in two main ways - as ends in themselves, for making stuff (like plastics) or for their chemical inputs (like fertilisers), where their chemical composition matters; or for carrying energy (oil, gas, coal, wood) where we are really just interested in releasing energy from breaking apart the bonds via combustion.
In this post, I break it into these two parts, tackling green chemistry first. This represents a smaller share of total emissions than emissions from fossil fuels used as energy carriers, but I’m taking it first as it takes prioritised as an end use for green molecules. I then look at the main options for green molecules as energy carriers and the (relatively few) use-cases where electrification will fall short and they will still be required .
Even by the standards of this series of posts this topic requires industrial-strength distillation, due to the highly heterogeneous nature of chemicals and fuels. Hopefully, it can serve readers as a reference and jumping off-point for further investigation, but at minimum I’d like you to leave with the following takeaways:
Round trip efficiency is a challenge. For both chemicals and fuels, decarbonising effectively requires pushing molecules energetically up-hill, so we need to be very mindful of allocating clean electricity generation to that endeavour where it might otherwise be used my effectively for decarbonising the grid or expanding electrification.
Harness nature. Bioresources are the cheapest way to get many green molecules, but sustainable supply is limited and, once again, shouldn’t be squandered on things which can otherwise be directly electrified.
External factors matter. Whilst we should aim for the most cost- and energy-efficient transition possible, ultimately it will evolve according to what people are willing and able to build. For example, green electricity might be the lowest hanging fruit from a technical perspective, but if new transmission can’t be permitted and interconnection queues are too long for renewable projects and subsidies are generous ($3 / kg for low-carbon H2 under the IRA), then we’ll see hydrogen get a bigger role than it “should”. (I don’t cover this point in the post, but it’s important context.)
Green chemicals:
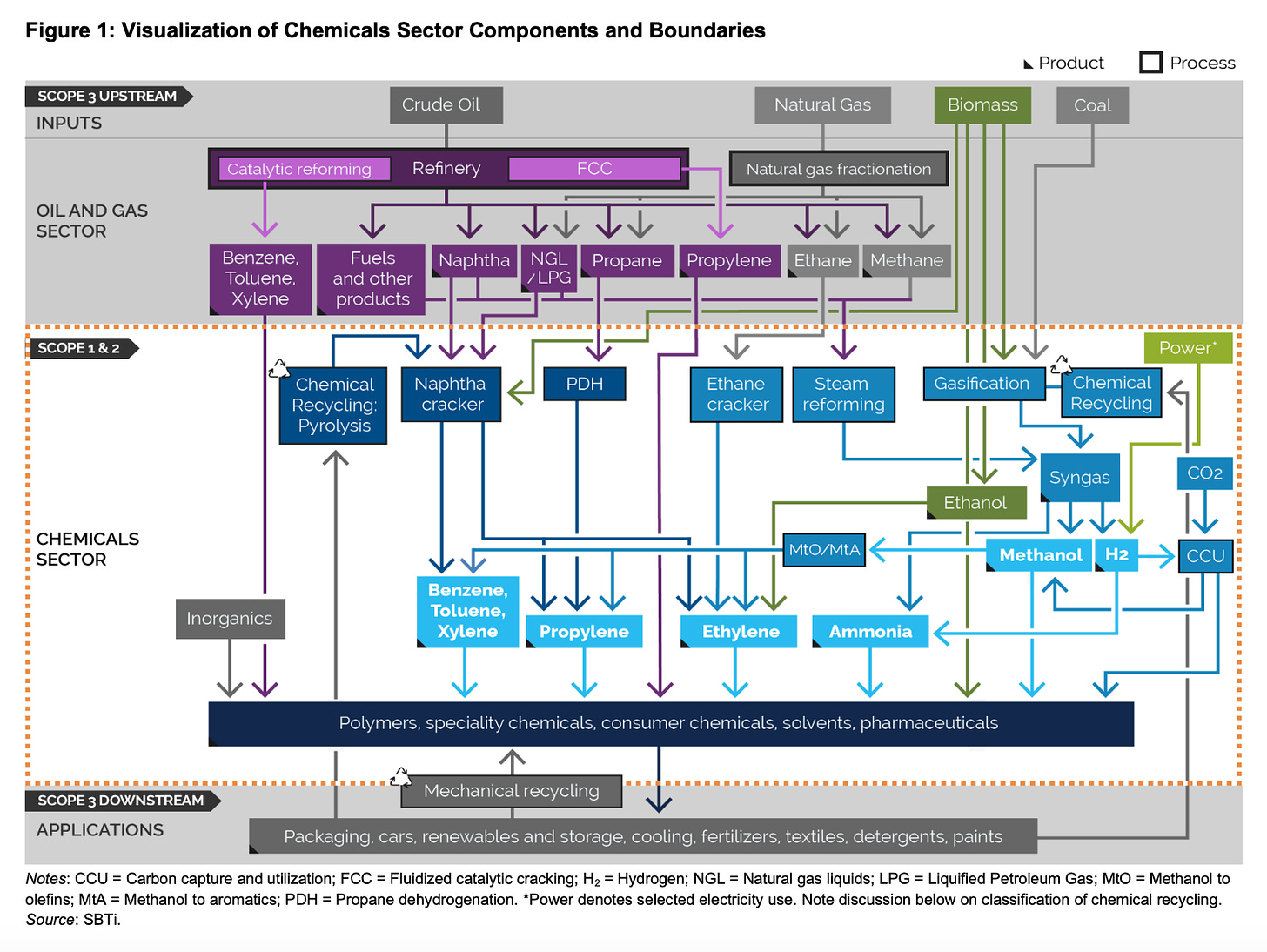
Whilst it isn’t very visible in our everyday lives, we are dependent on molecules produced by the chemical industry for many of our most basic needs, not least of all for fertiliser production, which is responsible for feeding half the people on the planet. The chemical industry is vast and its boundaries - and hence emissions - are difficult to define. Whilst the study cited by the IPCC in its latest report puts it at 2.8 GT, as the IEA defines it, it is just shy of 1 GT per year. The IEA figure includes ammonia, methanol, and what it terms “high-value chemicals” which are ethylene, propylene, benzene, toluene and mixed xylenes - all organic chemicals, or ones that are carbon-based and currently obtained from fossil fuel feedstock. The IEA leaves out big chunks of hydrogen production that are used for refining, and in steel production (a good use of green H2, but no climate benefit if it’s made the traditional, high-emission way). Hydrogen production alone represents almost 1GT of CO2 emissions / year. What we can definitely say is that there are significant emissions related to the molecules we need to make stuff, but it’s complicated - see below from SBTi’s Chemicals program, yikes!
Fossil feedstock: According to the IEA, fossil fuels are used as the feedstock for 70% of chemicals and the petrochemical industry (the bit that uses fossil feedstocks) accounts for 14% of oil demand and 8% of natural gas demand globally. As demands for stuff continues to rise whilst demand for oil in particular as a fuel drops because of electrification of transport, that proportion is likely to rise. As it happens, chemicals are a pretty good use of fossil fuels, much better than fuel. Why is that?
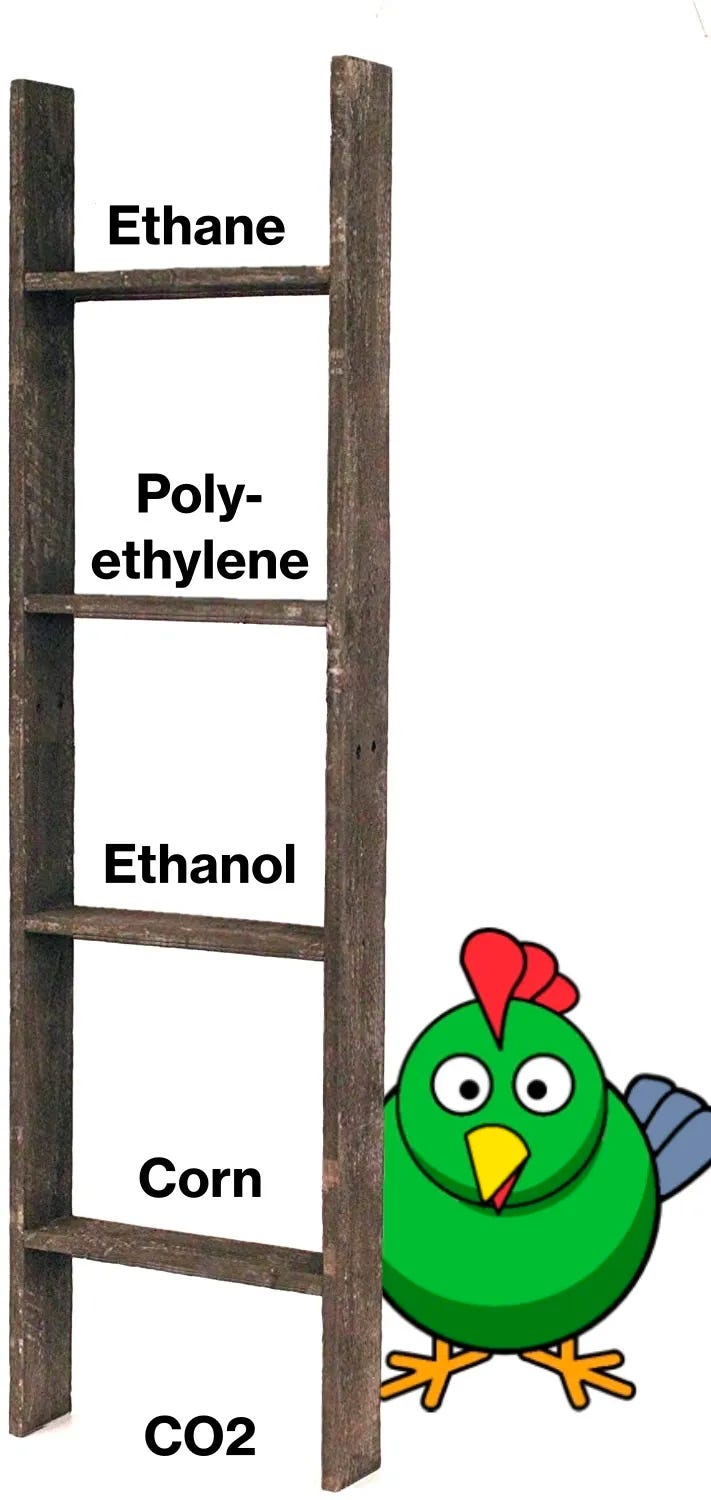
Inherent value of molecules and energy ladder: Unlike with fuels, which can be replaced in most cases with electrons (see exceptions in below section), when it comes to stuff the molecules are the point. When we are producing stuff we want to start with flexible feedstocks that can be relatively easily converted to valuable end uses. Here, I’ll borrow a simplified visualisation from Doomberg:
The implication of the above ladder is that where we want to produce high value organic molecules with higher energy content from very low energy CO2 or sugars, we need to inject extra energy to push them up the ladder. [Update: I’ve gotten some feedback from a process engineer - thanks Paul Martin! - that the linearity suggested by the Doomberg ladder is not absolutely accurate as it still takes a lot of energy to crack ethane into ethylene - the precursor to polyethylene - but that the broader point around fossil feedstock being an easier starting point to get to complex hydrocarbons holds. Plastics and materials are a better use of our fossil energy endowment than using them for fuel!]
So how do we go about decarbonising the valuable molecules we currently get from petrochemicals? The short answer is - with great difficulty. (Check out the complexity of tackling just a single value chain - work on decarbonising PVC from Center for Houston’s Future.) However, there are a couple of broad areas that we might consider.
Biological pathways: Whilst it is energetically expensive to push molecules up the ladder from CO2 or sugars (e.g. corn), it is relatively efficient via biological processes using enzymes (biological catalysts) or microbes. There are a number of companies looking to move either CO2 or sugars up the ladder to alcohol or beyond. To name a few - Lanzatech, Solugen, and Cemvita, each at various stages of development. Additionally, the Energy Transition Commission’s report on bioresources clearly states that bioresources should be prioritised not as fuel but as feedstock, both for timber and wood materials as well as an input for bioplastics to replace some petrochemical demand (although it notes that this technology needs to be advanced).
Energy efficiency: This might technically fit into the earlier post on efficiency and electrification to reduce the demand for fossil fuels, but there are a couple of companies worth mentioning specifically in the context of chemicals. The chemicals industry uses vast amounts of energy creating heat and pressure. There are companies looking to replace those processes with either membranes (Via Separations, Osmoses) or light (Syzygy Plasmonics - efficiency / different feedstocks).
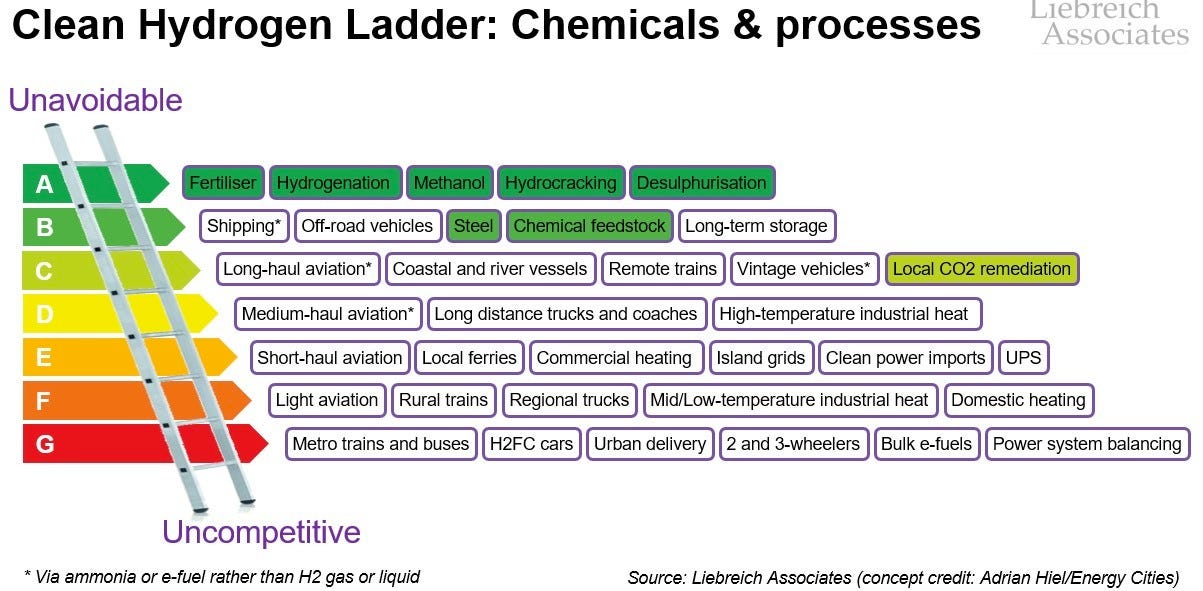
Hydrogen - decarbonising supply for existing uses: The current hydrogen market is about 90mm tons per year, and almost all of it is produced from fossil fuels (mostly steam methane reforming) producing almost 1GT of CO2 / year. It is used entirely for its value in chemical reactions and not at all as a fuel. The two biggest uses of hydrogen today are for ammonia production (the vast majority of which is used for fertiliser) and for refining, with some going to methanol production and to direct reduction of iron in steel making (more below). Hydrogen seems to be a weirdly emotive topic, but the top priority is replacing current uses with the low-carbon kind. For more on the priorities of low-carbon hydrogen, I’ll defer to Michael Liebreich’s Hydrogen Ladder, only to note that the entire top row and ⅖ of the second is for existing chemical processes:
Hydrogen in steel production: Hydrogen use for iron and steel production should be one of the higher priorities for new green H2 capacity. The sector is responsible for about 2.5GT of emissions, which includes both emissions from the heat sources and process emissions. According to the IEA, about 5 million tons of H2 is currently used in 7% of steel production, where it is mixed with carbon monoxide in various concentrations (syngas) to reduce iron ore to sponge iron. (“Reduce” in this context means removal of oxygen atoms, opposite of “oxidise”.) Emissions from this reduction process can be basically eliminated by using 100% hydrogen, which bonds with the oxygen from the iron ore to form water (H2O) instead of having carbon bond with it to make carbon dioxide. The most prominent project underway is H2 Green Steel in Sweden, which is going to take advantage of abundant local clean electricity (hydro & wind) to use hydrogen for the reduction and electricity as the heat source. Just to note, one other (non-hydrogen) potential pathway to decarbonise steel is through the direct electrolysis process that is being developed by Boston Metal. Chris Goodall of Carbon Commentary did a useful comparison of Boston Metal’s approach and the hydrogen DRI approach. Rob West also recently looked at it here.
Reducing ammonia demand: as well as replacing high-emitting chemical fertiliser with a low-carbon equivalent, we should make efforts to reduce overall demand for chemical fertilisers either with regenerative ag practices that put nitrogen back in the soil (e.g. rotating crops with legumes) or using biological additives for putting nitrogen in the soil (as yet unproven, but plenty working on it - Switch Bio, Pivot Bio, Kula Bio, etc), or other novel ways of adding nitrogen (e.g. Nitricity - non-ammonia fertiliser). Judicious use of fertiliser is also critical because it creates nitrous oxide (NO2) which represents roughly 3GT CO2e and also is messing up the earth’s natural nitrogen cycle.
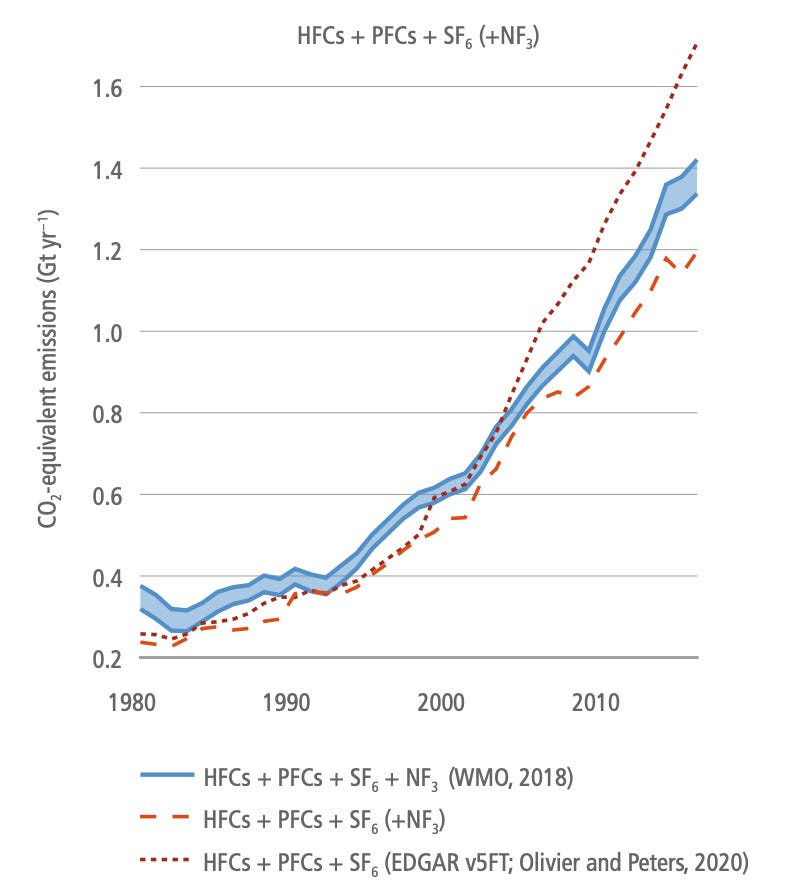
Refrigerants and other super-GHGs: Whilst not a major contributor compared to carbon dioxide or methane, fluorinated gases (or F-gases) still represent about 1.5 GT CO2e emissions per year and that number has grown about 5x since 1990 (see below chart). Their growth represents a classic case of humanity replacing one problem with another when hydrofluorocarbons took over following the ban of ozone-depleting CFCs under the Montreal Protocol. As usual, The Simpsons have a quote for that.
Whilst F-gases are used in small quantities, their global warming potential runs to thousands of times that of CO2 and they tend to be long-lived. The vast majority of F-gases are used as refrigerants but also in industrial processes like making of foam. Replacing these gases would have a massive climate impact - Project Drawdown estimates there is something like 45GT of CO2e worth of abatement on the table over the 30 years to 2050. Climate-friendly cooling deserves (and will get) its own dedicated post, but there are a number of people working on alternatives that don’t use F-gases such as Gradient. Luckily, there are lots of alternatives available including ammonia, propane and even carbon dioxide (longer list here for anyone interested). In this group of F-gases is also sulphur hexafluoride (SF6), which is the most potent greenhouse gas there is at about 23,000x the global warming potential of CO2. It is currently used in components of the electrical grid, but there are people working on it, including both incumbents and German start-up Nuventura.
Green molecules for carrying energy:
The vast majority of the molecules we use today are in the form of hydrocarbons as energy carriers. Whilst the first order of business is to reduce the demand for them through energy efficiency and electrification, there will remain some energy end-uses that are resistant to electrification. This is usually down to the requirement for energy density - either volumetric (energy / unit volume) or gravimetric (energy / unit weight). For these we will ultimately need to be able to decarbonise these energy-carrying molecules. We can produce these through a few principal pathways.
Bioenergy / biofuels: These represent pretty much the entirety of non-fossil molecule energy use today. However, whether or not that bioenergy is low-carbon depends on the particular case. Biofuels have the capacity to be low-carbon because, whilst carbon is put into the atmosphere when they are combusted, it was only recently taken out through photosynthesis. But this requires that the feedstock is sustainably sourced. Still today the largest use of biomass for energy (about 35%) is what the IEA calls “traditional use”, i.e. burning wood for cooking. According to the Clean Cooking Alliance, cooking with wood and charcoal represents 1 GT of carbon emissions and about a third of that is sourced unsustainably, i.e. by chopping down forests, with the associated biodiversity impacts on top of the climate ones. Whilst the IEA estimates that the world currently uses about 70 EJ of bioenergy, the Energy Transition Commission suggests that there is only about 65 EJ worth of sustainable bioenergy available, of which 40 EJ should be prioritised for materials and feedstock for chemicals (as noted above), leaving only 25 EJ for energy. That is about 7000 TWh of primary energy or around 5% of today’s primary energy use. These should be prioritised for those applications that require liquid fuels. Bioenergy is turned into energy-dense liquid fuels for those niche applications that require them, either through fermentation (e.g. corn or sugarcane ethanol), or through gasification and then through a fischer-tropf process to turn it from gas to liquid. The other way bioresources can contribute to the energy mix is via biogas. This can be produced via agricultural residues, animal manure, or gases from landfill or waste water treatment. The European Biogas Association estimates that by 2050 Europe could produce the equivalent of 40% of its 2022 natural gas demand via biogas. To be sustainable, biomass (as feedstock or energy carrier) should be sourced adhering to the below principles:
Hydrogen as fuel: The appropriate role for hydrogen as an energy carrier has been vastly overstated in the press and, unfortunately, in some government policies. As already mentioned (but merits repeating), we already have a 1GT abatement opportunity to replace the 90mm tons of today’s high-emission hydrogen with the low-emission variety. Hydrogen does have the advantage of being energy dense on a gravimetric basis (joules / kg), but the other side of that coin is that, because it is so light, it is not dense at all on a volumetric basis. That makes it very expensive to transport, along with other factors such as leakage and high-boil off rates when it is liquified (which also takes a huge amount of energy). Michael Liebreich’s piece The Unbearable Lightness of Hydrogen covers this well. Really the core underlying challenge with hydrogen as a fuel is that, with production and all of the other steps around making it useful, it is really inefficient, coming out at something like 3-5x less efficient than using electricity directly, depending on the application. Very briefly on the supply side, it is easy to get lost in the hydrogen rainbow of different hydrogen production pathways. The key thing is low-carbon! Generally that means using renewable electricity, but it could also mean methane pyrolysis (turning natural gas into H2 and solid carbon) like Monolith, using heat + power from nuclear fission, mining natural hydrogen reservoirs, using fossil fuels with carbon capture (although methane leaks make this challenging to be truly low carbon), or training microbes to eat crude oil to produce hydrogen like Cemvita is doing.
Electro-fuels / e-fuels / synthetic fuels: Most often these refer to hydrocarbons synthesised from low-carbon hydrogen and CO2, from point source capture (ideally from sustainable biogenic sources if it is to be truly low-carbon) or, eventually, direct air capture (DAC). The inefficiency of using hydrogen applies to e-fuels, only more so, as there are additional steps. However, whilst e-fuels have a disadvantage compared to hydrogen on energy efficiency, their big advantage is drop-in compatibility with existing infrastructure and ease of transport / storage.
Ammonia: somewhere in between hydrogen and e-fuels sits ammonia - somewhat more efficient than e-fuels, somewhat easier to transport than hydrogen - but it comes with its own drawbacks of being highly toxic and emitting nitrous oxide (a potent GHG) when burnt. Carbon Direct recently put out a report on ammonia’s role for decarbonisation.
As you can garner from the above, scaling green molecules as energy carriers is no mean feat. We quickly run into issues around availability of sustainable feedstock (biofuels) or major challenges around energy efficiency and infrastructure buildout. That reality seems to be sinking in, as we observe the putative jurisdiction of green molecules as energy carriers is gradually shrinking. Hydrogen cars are dead. Hydrogen for residential heating is a non-starter. Hydrogen for long range trucking seems to be on the out also - Scania publicly ditched their hydrogen efforts a couple of years ago, hydrogen truck companies Hyzon and Nikola have both flamed out, and Tesla is gradually starting to ramp up production of the Tesla Semi. Remaining end applications will (or at least should!) be confined to the relatively narrow cases where a lot of energy needs to be carried over very long distances; namely - shipping and, in particular, aviation.
Aviation: Only about 5% of aviation emissions are going to be able to be abated through electrification as the range of electric planes will cap out at about 400km over the next 10 or so years, extending maybe to 600km by 2050. This means that the vast majority will need to be addressed with green molecules. Liquid fuels that can be blended with conventional jet fuel (“jet-A”) are broadly referred to as sustainable aviation fuel (“SAF”), which could be either biofuels or e-fuels. All of the SAF produced today falls into the biofuel bucket and currently pretty much all of that is converting used cooking oil. Another option is to use household rubbish (municipal solid waste or “MSW”), which is what Fulcrum is doing. SAF is also being pursued by the synbio companies mentioned above, again, because it is the highest value fuel. The e-fuels side is further behind, but a number of companies are working on it including MetaFuels, Infinium and Ineratec. Using hydrogen directly with fuel cells to power electric drive trains is another option (e.g. Zero Avia). This is less profligate from an energy perspective than e-fuels but it does require new infrastructure and, critically, redesigned planes. Because hydrogen is less volumetrically dense than jet-A, it requires planes with longer fuselages to store it. However, because it is more gravimetrically dense, it gives something back on the amount of weight that needs to be lifted from the ground. Another potential advantage of alternative fuels other than fossil Jet A is the potential to reduce contrails. Amazingly, the climate impact of contrails, which occur in about 1 in 20 flights and last for half a day, is about equivalent to the 100-year impact from the CO2 released by all flights. (This surely means that finding a way to reduce contrails must be one of the highest leverage climate actions out there?) I covered the challenge of decarbonising aviation in more detail in a previous post, and I would highly recommend this recent podcast with Rob Miller of Cambridge’s Whittle Laboratory.
Shipping: Like aviation, there is low-hanging fruit for electrification on shorter routes but a much greater challenge electrifying long-distance shipping. There is though one company that deserves a special mention that is taking an innovative approach to long distance electrification, which is Fleet Zero. Long distance shipping’s challenge is a bit different from aviation in that space rather than weight is at the greater premium. It is also used to paying very little for fuel as shipping uses fuel oil or bunker fuel, which is quite literally the bottom of the barrel, the tar-like residual that is left from crude oil after refining, so everything else is much more expensive by comparison. In general though, the discussion is centred around either ammonia or methanol, both of which are more volumetrically dense than hydrogen. Methanol has some advantages over ammonia in that it is more stable (easier to store or “bunker”), less toxic, and can be used in existing engines. It seems to have stolen an early lead with Maersk placing orders for 19 dual fuel ships that can run on methanol and supporting the development of new e-methanol production facilities. The advantage of ammonia over methanol from a decarb perspective is that, well, it has no carbs. So for methanol to be truly decarbonised it would need to have point-source capture + storage (Carbon Ridge is working on that) or be e-methanol.
Long duration storage: There doesn’t seem to be a consensus on this one, but transforming excess electricity into molecules is a highly scalable way to store energy over long periods. That could involve storing hydrogen in salt caverns, or converting into methane which fits within existing energy infrastructure (e.g. Electrochaea) or another derivative like ammonia.
Other - miscellaneous: There are other niche applications for green molecules as energy carriers either already in use (e.g. forklifts) or under development (back-up generators, mining equipment), but they don’t represent large chunks of energy demand or emissions. One example I came across recently, which blew my mind - Japan is planning on using ammonia as a fuel to burn in modified thermal power plants, allowing them to extend the life of their existing coal fleet. This is a great example illustrating that the particularities of politics / geography / infrastructure will mean that there will be a far more heterogeneous landscape than energy economics or engineering by itself would suggest.
At the end of the day, scaling up the production of green molecules to the point of climate relevant is going to be incredibly challenging. It will require the vast build out of new infrastructure, both so we can achieve the energy abundance where it makes sense to push molecules up the energy ladder and then distribute / store / use them. It also will require the orchestration of large and embedded systems. The truth is that we are going to be using fossil fuels as chemical inputs and energy-carriers for a long time to come. This requires that we scale carbon management infrastructure across the energy system to reduce the flow of greenhouse gases (decarbonising hydrocarbon supply and point source carbon capture) and then the stock (carbon dioxide removal). And that will be covered in the next and final instalment of this series.